Structure and function of Gfat-1 elucidated
Next steps on the road to drug development
With age, more and more protein fragments accumulate in cells, proteins "aggregate" and the risk to suffer from diseases such as Alzheimer's or Parkinson's increases. Six years ago, researchers from the Max Planck Institute for Biology of Ageing found a protein in the small worm Caenorhabditis elegans, known as GFAT-1, which activates the degradation of damaged proteins and is an interesting target for the development of drugs. Martin Denzel's research group has now achieved further important steps: they were able to elucidate the structure of the protein and show that the protein is also important for the degradation of protein aggregates in mammalian cells.
Publishing a discovery is not the end of the project for researchers. In many cases, this is when the work really begins. After their finding about the positive effects of GFAT-1 in nematodes, Martin Denzel and his team still had many open questions. "In the long term, we want to find a drug that helps prevent neurodegenerative diseases. To this end, we are looking for active substances that improve the function of GFAT-1. However, it was still completely unclear how exactly GFAT-1 is activated and whether GFAT-1 also plays a role in other organisms," explains Denzel.
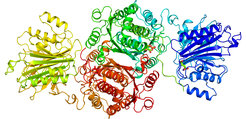
Crystal structure of GFAT-1
In order to clarify the structure of GFAT-1, the researchers in Denzel's laboratory, in collaboration with Ulrich Baumann at the University of Cologne, used protein crystallography. In this method, proteins are irradiated with intense X-ray light to decipher their structure and function. Sabine Ruegenberg was able to analyse the protein in its entire length and show how the activation of the protein works. "This is a breakthrough. Now we can search for active substances in a much more targeted way," says Denzel.
Protein aggregates in mammalian cells
As a next step, Moritz Horn investigated the effect of GFAT-1 in mammalian cells. To this end, they induced the formation of harmful protein aggregates in mouse cells. Interestingly, they observed that in cells with activated GFAT-1, the aggregates were greatly reduced. "These are very promising results that will help us make significant progress on the road to drug development. However, we are still a long way from reaching our goal," said Denzel.
The research was carried out in cooperation with Adam Antebi and Sarah Denzel from the Max Planck Institute for the Biology of Aging and Ulrich Baumann from the Institute of Biochemistry at the University of Cologne.
Learn more about the work in the Denzel lab.








